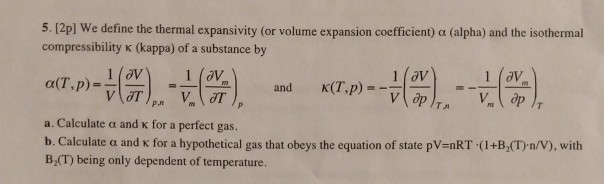![SOLVED: [6 marks] This problem gives practice with taking derivatives! The isothermal compressibility Kr (kappa) describes how the volume of a substance changes with pressure at constant temperature. Given Page of 3 SOLVED: [6 marks] This problem gives practice with taking derivatives! The isothermal compressibility Kr (kappa) describes how the volume of a substance changes with pressure at constant temperature. Given Page of 3](https://cdn.numerade.com/ask_images/8fd0309efe92472f8d87f1bd77df5223.jpg)
SOLVED: [6 marks] This problem gives practice with taking derivatives! The isothermal compressibility Kr (kappa) describes how the volume of a substance changes with pressure at constant temperature. Given Page of 3

Density, Speed of Sound, Compressibility and Related Excess Properties of Methane + n-Heptane at T = 303.15 K and p = 10 to 70 MPa | SpringerLink
![Calculated isothermal compressibility κT\documentclass[12pt]{minimal}... | Download High-Resolution Scientific Diagram Calculated isothermal compressibility κT\documentclass[12pt]{minimal}... | Download High-Resolution Scientific Diagram](https://www.researchgate.net/publication/329439239/figure/fig3/AS:961808689008649@1606324469311/Calculated-isothermal-compressibility-kTdocumentclass12ptminimal_Q640.jpg)
Calculated isothermal compressibility κT\documentclass[12pt]{minimal}... | Download High-Resolution Scientific Diagram

The compressibility kappa of a substance is defined as the fractional change in volume of that substance for a given change in pressure : kappa = - 1V dVdP (a) Explain why
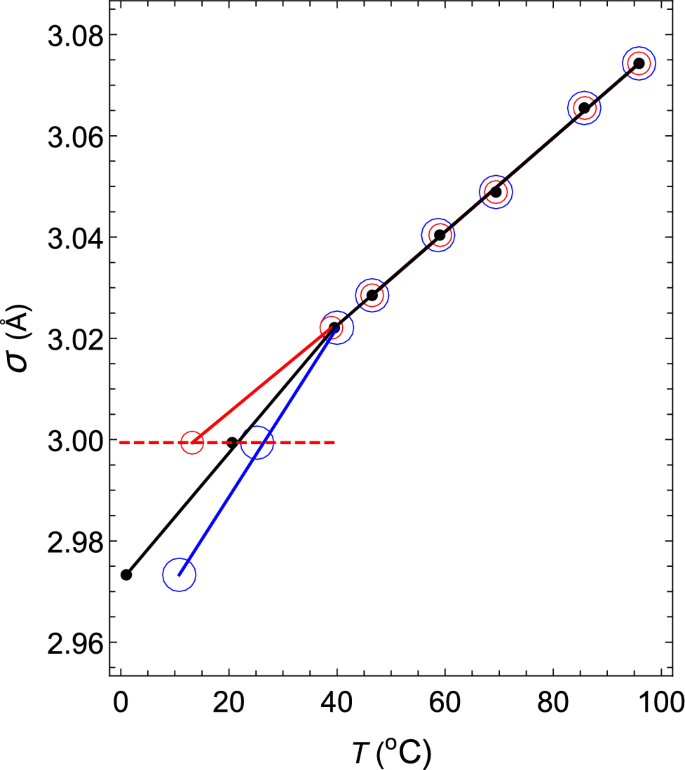
Thermodynamic mechanism of the density and compressibility anomalies of water in the range − 30 < T (°C) < 100 | Scientific Reports
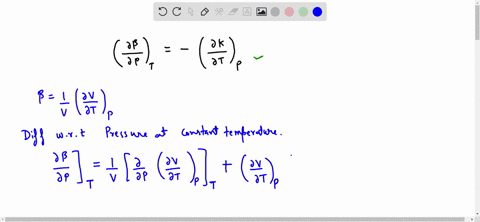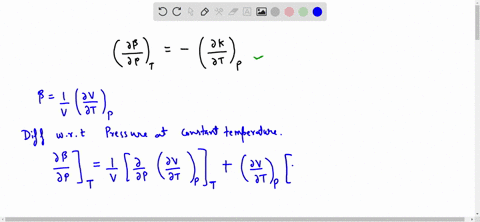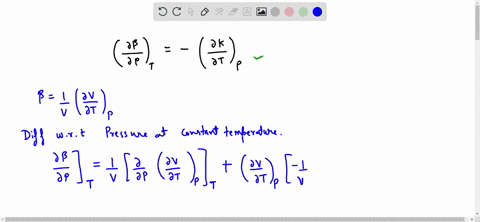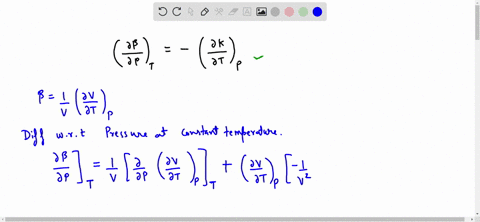
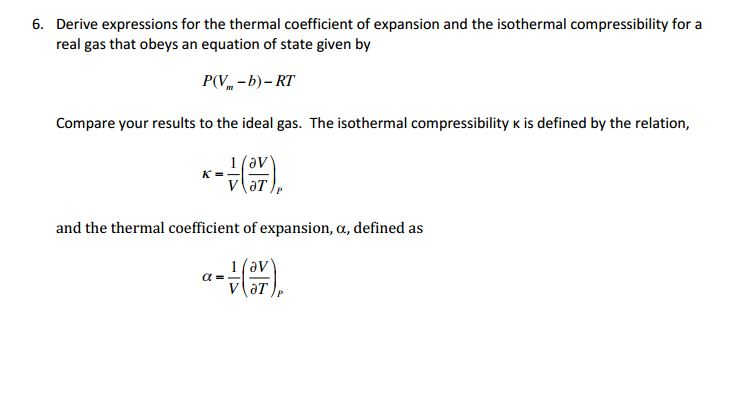
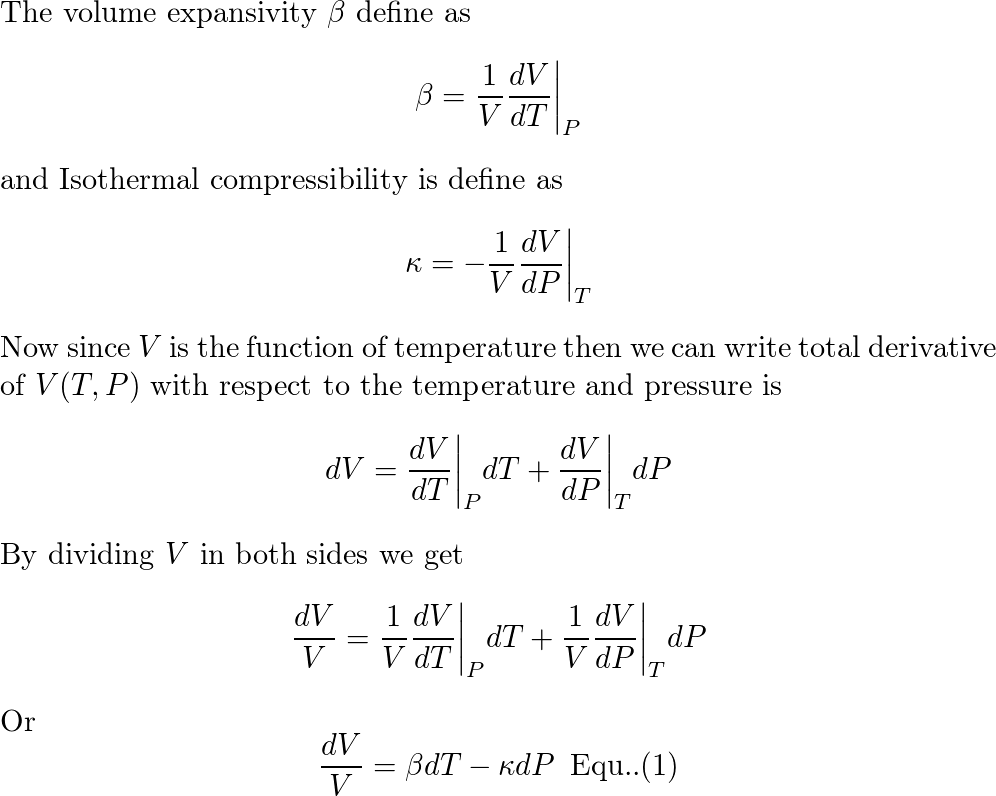
SOLVED:Generally, volume expansivity βand isothermal compressibility κdepend on T and P. Prove that: ((∂β)/(∂P))T=-((∂κ)/(∂T))P
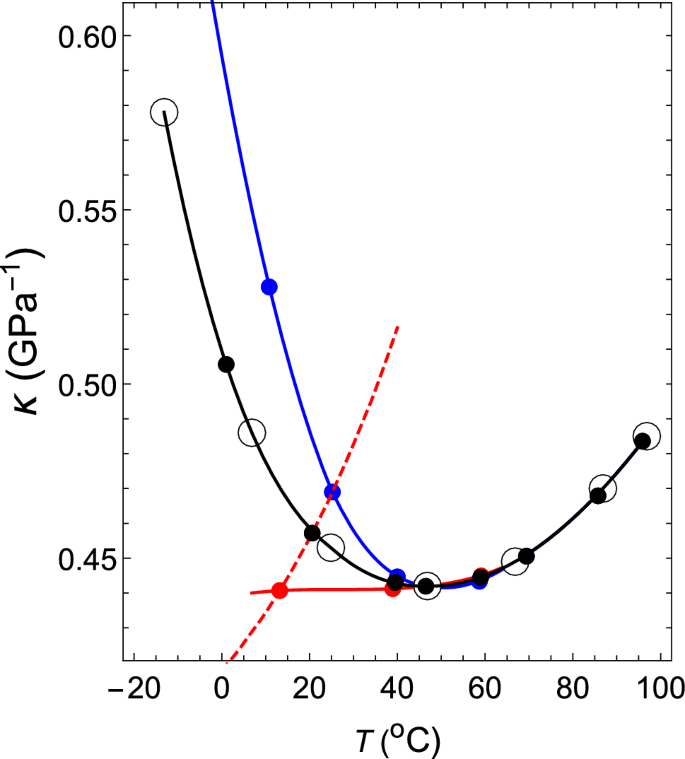
Thermodynamic mechanism of the density and compressibility anomalies of water in the range − 30 < T (°C) < 100 | Scientific Reports
![PDF] Anomalies in isothermal compressibility and exponent of pressure in spin-orbit-coupled degenerate Fermi gases | Semantic Scholar PDF] Anomalies in isothermal compressibility and exponent of pressure in spin-orbit-coupled degenerate Fermi gases | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c7385b47eef5baa94554ca8fef1d7d1d2db20349/1-Figure1-1.png)
PDF] Anomalies in isothermal compressibility and exponent of pressure in spin-orbit-coupled degenerate Fermi gases | Semantic Scholar

Define compressibility factor, z and explain how its value evolves with pressure and temperature. | Homework.Study.com
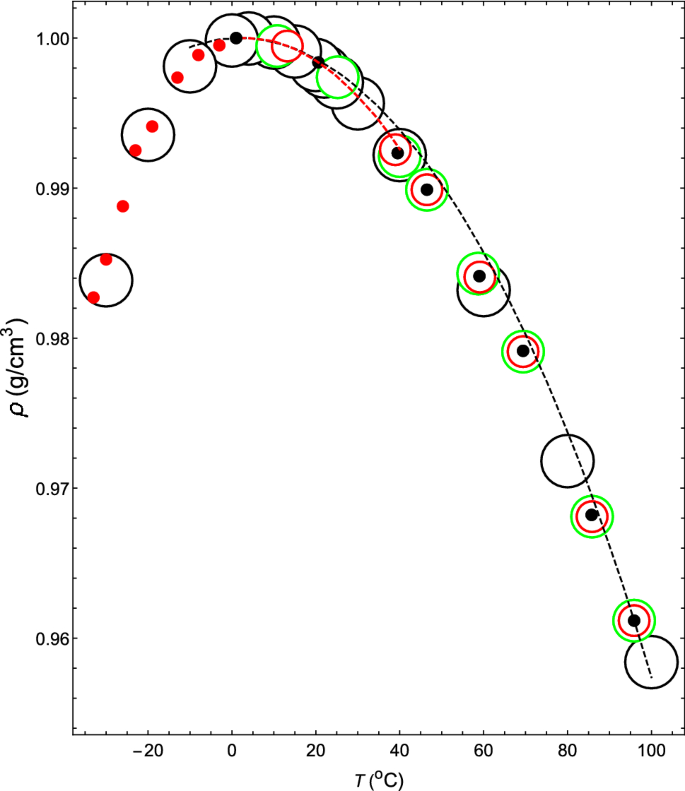
Thermodynamic mechanism of the density and compressibility anomalies of water in the range − 30 < T (°C) < 100 | Scientific Reports
![Calculated isothermal compressibility κT\documentclass[12pt]{minimal}... | Download High-Resolution Scientific Diagram Calculated isothermal compressibility κT\documentclass[12pt]{minimal}... | Download High-Resolution Scientific Diagram](https://www.researchgate.net/publication/329439239/figure/fig3/AS:961808689008649@1606324469311/Calculated-isothermal-compressibility-kTdocumentclass12ptminimal.png)


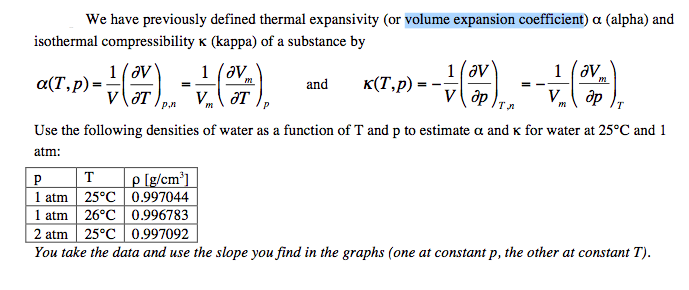




![PDF] Isothermal compressibility determination across Bose-Einstein condensation | Semantic Scholar PDF] Isothermal compressibility determination across Bose-Einstein condensation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dee6eb40284e9a1c21db48092251ce29b6426391/3-Figure1-1.png)