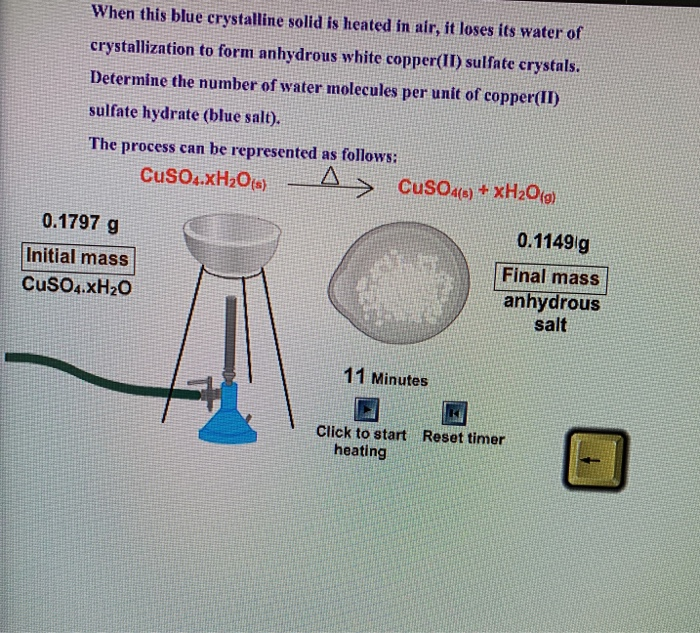
The hydrated salt Na2CO3.xH2O undergoes 63% loss in mass on heating and becomes anhydrous. The value of x is: (Gram atomic mass : Na = 23; C = 12 ; O = 16 )

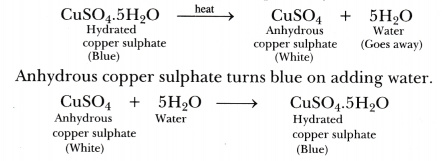
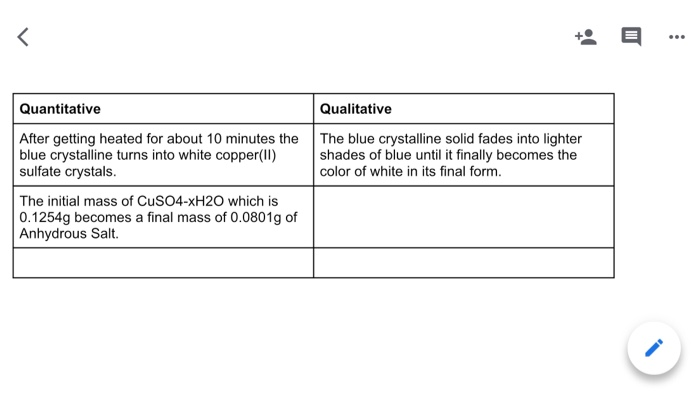
Give suitable chemical terms for the following:A definite number of water molecules bound to some salts.
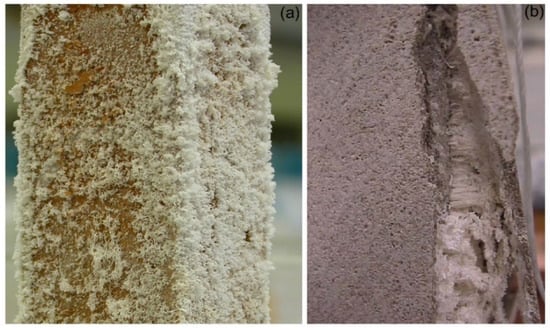
Molecules | Free Full-Text | Molecular Crystallization Inhibitors for Salt Damage Control in Porous Materials: An Overview | HTML

A sample of blue crystalline salt X is heated in a clean dry hard glass test tube . The salt on - Brainly.in

On strongly heating, a blue salt leaves a black residue. Which of the following cations can be present in the salt?

the crystallisation salt na2so4xh2o on heating losses 559 of its weight the formula of the crystalline salt is - Chemistry - TopperLearning.com | cf614k66