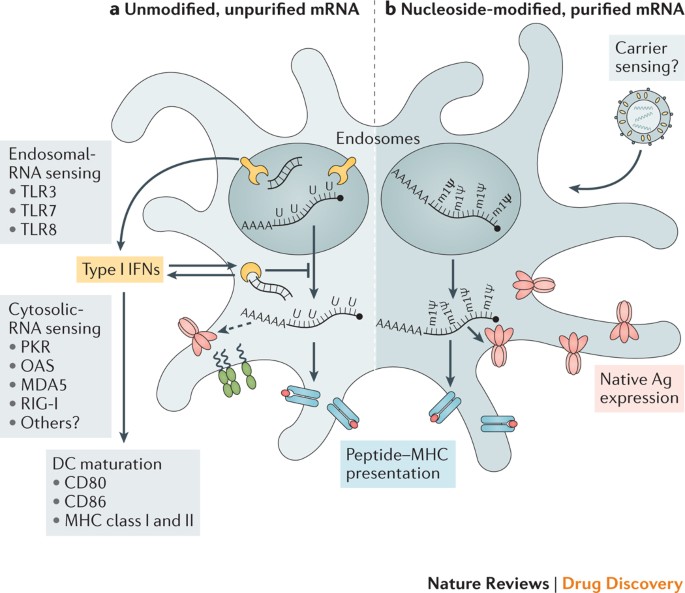
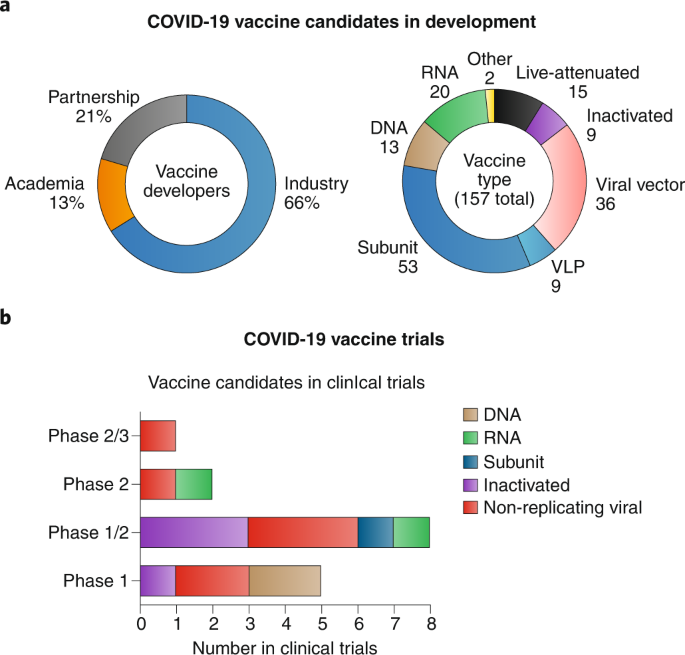
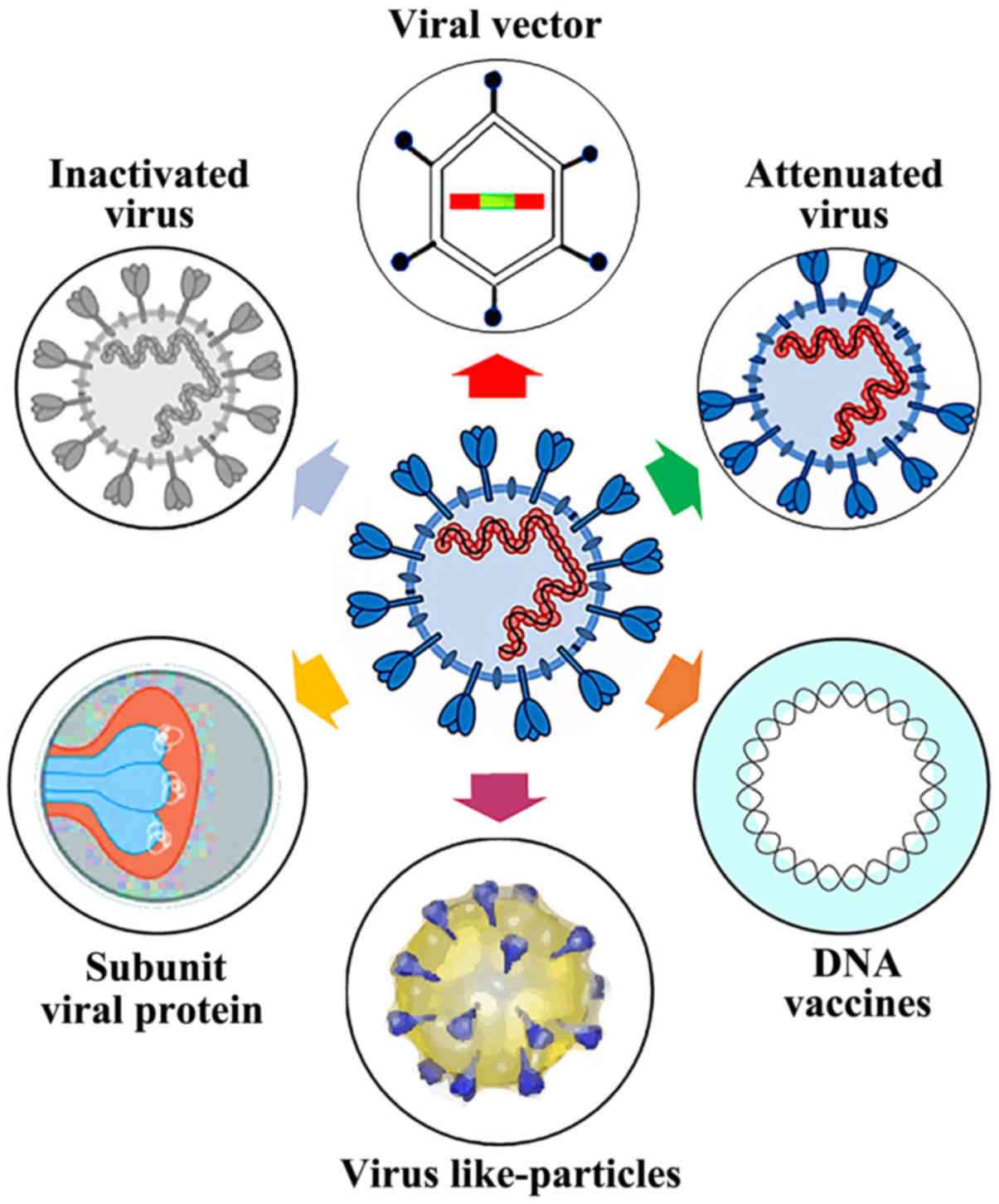
Current Status of COVID‐19 (Pre)Clinical Vaccine Development - Ye - 2020 - Angewandte Chemie International Edition - Wiley Online Library
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-C

Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses: Molecular Therapy

Michael J. Hogan's research works | The Children's Hospital of Philadelphia, PA (CHOP) and other places

COVID-19 tracker: Regeneron's antibody cocktail hit by safety concerns; Novo's Rybelsus emerging from pandemic slump | FiercePharma
PDF) Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses