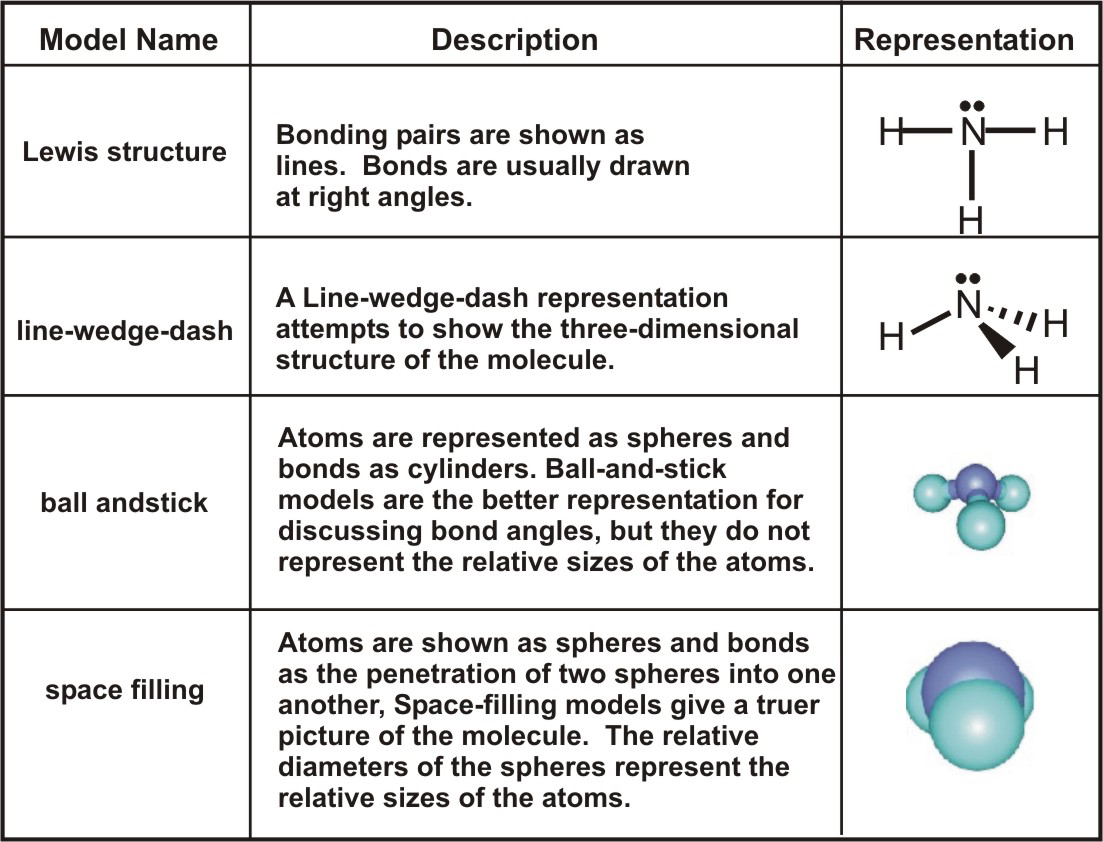
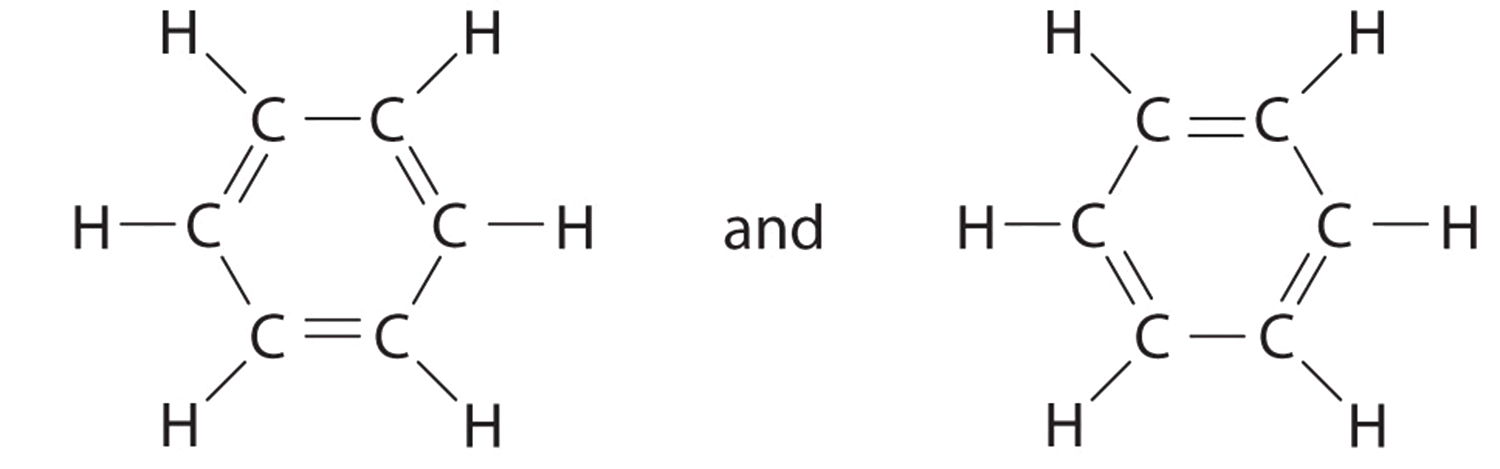Lewis Dot Structures Lewis Dot structures are used to represent the valence electrons of atoms in covalent molecules Dots are used to represent only the. - ppt download

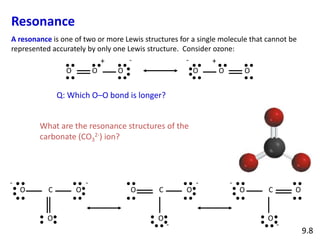
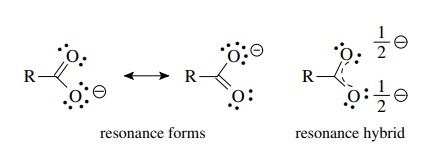
Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?

Question 44 Explain why CO 32- ion cannot be represented by a single Lewis structure. How can it be best represented?

Basic Concepts of Chemical Bonding Chapter 8. Three Types of Chemical Bonds Ionic bond Ionic bond –Transfer of electrons –Between metal and nonmetal ions. - ppt download
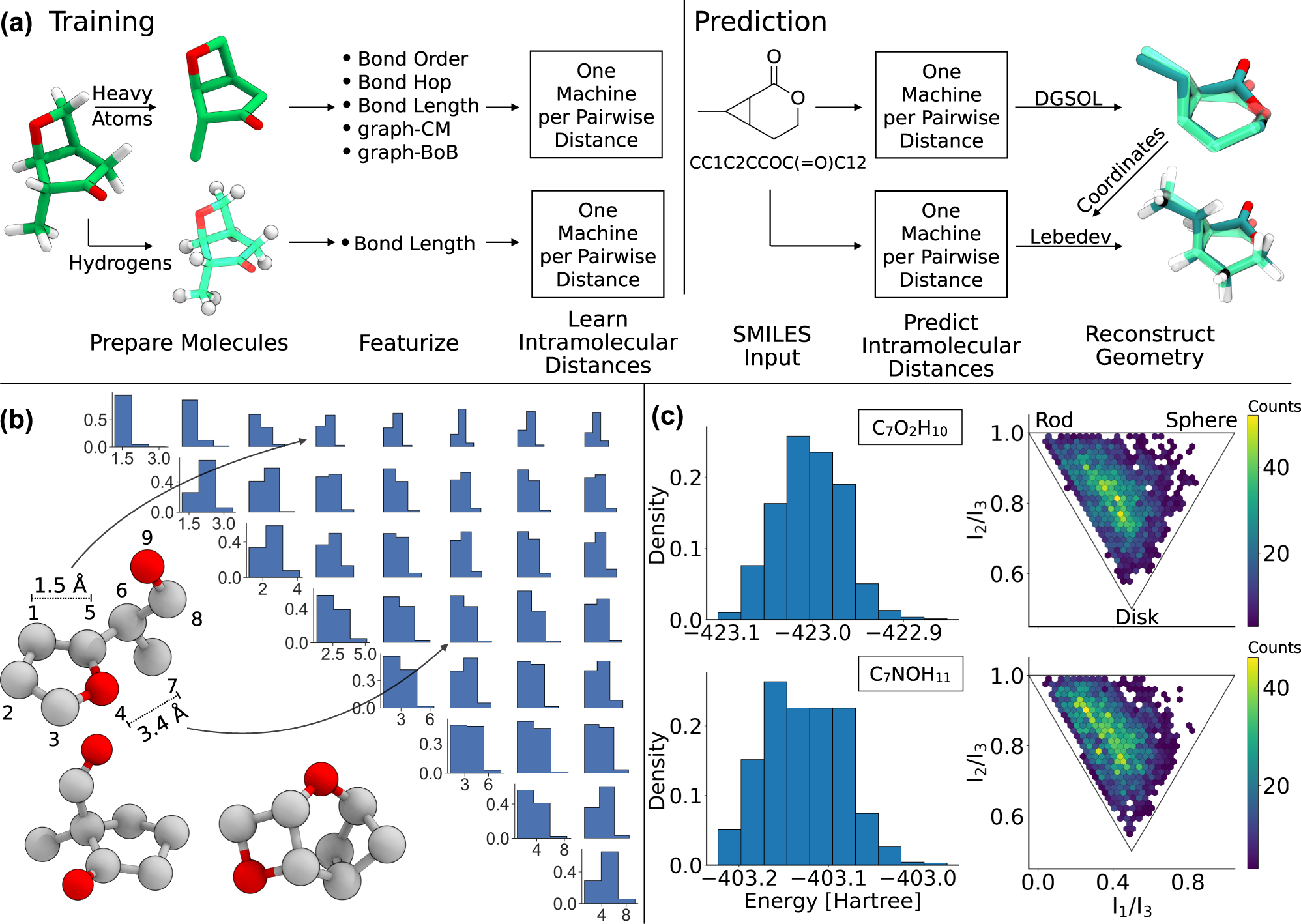
Machine learning based energy-free structure predictions of molecules, transition states, and solids | Nature Communications

Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Simplified S 0 singlet PES at the G3SX//M06-2X/aug-cc-pVTZ level of... | Download Scientific Diagram







/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)